behind-the-scenes of pqq
Many commonly known vitamins, such as vitamin C and vitamin B2, function as “redox-active” cofactors—a group of small molecules needed for catalyzing chemical conversions that drive the reactions happening in our bodies. Vitamin C, for example, is an antioxidant that participates in the redox cycle, helping to remove the toxic reaction by-products within the cell. Like vitamin C, PQQ is also an enzyme cofactor that has redox properties. Studies have shown that PQQ lowers the risk of disease during aging via stimulating important cellular activities. Understanding how PQQ behaves in nature helps to unlock the molecular basis of the beneficial effects of PQQ.
Oral supplementation of PQQ improves reproduction and enhances neonatal rates of growth compared with the response from diets devoid of PQQ. More recently, dietary supplementation of PQQ Na2 in broiler chicks has been shown to improve growth performance, carcass yield, immunity, and plasma status.
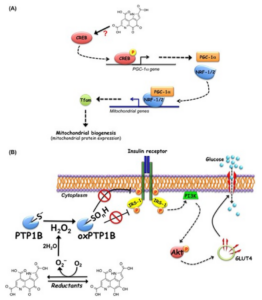
PQQ supplementation reverses the mitochondrial alterations and metabolic impairment and significantly improves the lipid profile in diabetic UCD-T2DM subjects.
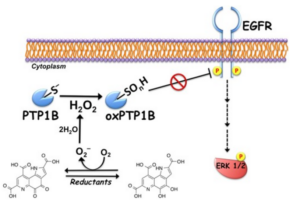
More recently, we found that PQQ elicits the ligand-independent activation of insulin signaling by inhibiting cellular PTP1B and enhances glucose uptake through the translocation of glucose transporter 4 in subjects C2C12 myotubes. In addition, we demonstrated that oral administration of PQQ for two weeks improved impaired glucose tolerance in type 2 diabetic KK-Ay subjects. Our findings clearly suggest that PQQ can be useful in anti-diabetic treatment for T2DM subjects.
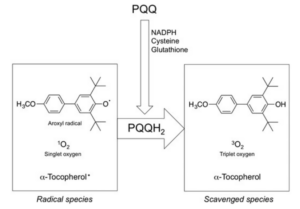
PQQ is reduced easily to PQQH2 by reaction with reducing agents such as NADPH, sodium borohydride, glutathione, or cysteine. A couple of in vitro studies demonstrated that the reduced form of PQQ (PQQH2) exhibits anti-oxidative capacity. The aroxyl radical-scavenging activity of PQQH2 was 7.4-fold higher than that of vitamin C, which is known as the most active water-soluble anti-oxidant. The singlet oxygen-quenching activity of PQQH2 was found to be 6.3-fold higher than that of vitamin C. Interestingly, PQQH2 works as catalyst in the singlet oxygen-quenching reactions. Moreover, it has been clarified that PQQH2 may rapidly convert two molecules of α-tocopheroxyl radicals to α-tocopherol. These results indicate that the pro-oxidant effect of α-tocopheroxyl radicals is suppressed by the coexistence of PQQH2. The summary of the radical quenching reaction is shown below.
Nerve growth factor (NGF) is a small protein that plays a critical role in the growth, development, and maintenance of neurons. PQQ has been found to promote NGF production, with one study showing a 40-fold increase in NGF. Increased levels of NGF help to maintain the health of the brain and peripheral nerves and stimulate nerve regeneration.
Neurons are susceptible to receive lethal damage from oxidative stress. This neuronal death is regarded as a cause of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. The ability of PQQ Na2 to protect human neuroblastoma SH-SY5Y cells from oxidative stress by 6-OHDA or H2O2 was tested. The cell viabilities were recovered in a dose-dependent manner by adding PQQ Na2. The inhibitory activity of PQQ Na2 was much higher than that of vitamin C or vitamin E at the concentrations tested. These results suggest that the protective effect of PQQ on 6-OHDA or H2O2-induced neurotoxicity is involved in its function as a radical scavenger, especially 
